What is a photon particle
What is a photon particle
What is a photon particle
What is a Photon: Particle or Wave?
Part 1
Evidence:
1) How a photon is created or absorbed.
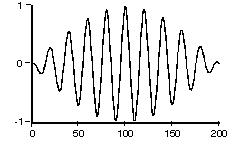
This should effectively be the form of a photon in space. The effective length for a S2 to S1 transition photon should be approximately: 12.2 cm to 122 cm (average = 61cm) based on their estimates.
» The state of affairs has been greatly influenced by over 40 years of popular belief that since a bound system exhibits only certain discrete energies and a transition from one to another cannot proceed through any observable intermediate levels, then the corresponding wave function must also evolve in a similar discontinuous manner. This interpretation has been shown to be incorrect. «
They go on to say:
At this point, the natural questions of the student are, «How is a photon created or absorbed? What is the mechanism of this process and how long does it take?» The usual instructor response may be that a transition involves a quantum jump, which is an instantaneous process and the Uncertainty Principle prohibits us from observing or describing in classical terms the details of the transition, or he/she may evade the question by claiming the concepts are beyond the scope of an introductory course and will be developed later in quantum physics or physical chemistry. After completing a bachelor’s degree, our student has been exposed to a lot of the prescriptive formalism of quantum mechanics with heavy emphasis on finding eigenvalues and solutions to the time-independent SchrГ¶dinger equation and possibly modest exposure to the time-dependent equation and perturbation theory for the purpose of developing transition probabilities. However, to her/his great disappointment, freshman questions probably still remain unanswered.»
They then explain how the actual transition has been observed in detail:
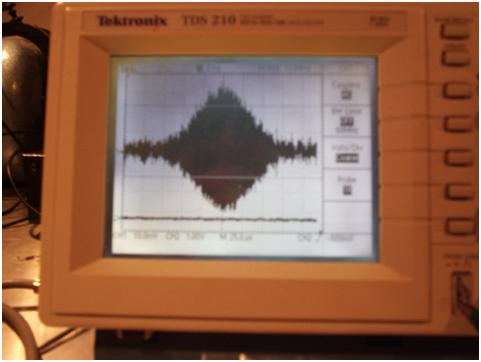
Fig.3: Spin Echo from a glycerol H1 sample at 18 MHz.
The radiative lifetime of an excited electronic state e.g. in a laser gain medium is the lifetime which would be obtained if radiative decay via the unavoidable spontaneous emission were the only mechanism for depopulating this state. It is given by the equation.
which shows that high emission cross sections and a large emission bandwidth inevitably lead to a low radiative lifetime. This is because the cross sections describe not only the strength of stimulated emission but also that of spontaneous emission. Another important aspect is that a shorter mean wavelength of the emission implies a shorter radiative lifetime. This results from the increased mode density of the radiation field. A consequence is that ultraviolet lasers tend to have a higher threshold pump power than infrared lasers.
The radiative decay rate of a classical electron oscillator is given by :
where n is the refractive index of the medium in which the oscillator is embedded, and the oscillation frequency is measured in Hz. A useful rule of thumb is that the purely radiative lifetime of a classical oscillator is approximately given by:
The Fig. 6 equation is used to calculate the photon length at different classical oscillator emission wavelengths as shown in the table below:
What is a photon?
The fundamental particle of light is both ordinary and full of surprises.
What physicists refer to as photons, other people might just call light. As quanta of light, photons are the smallest possible packets of electromagnetic energy. If you are reading this article on a screen or a page, streams of photons are carrying the images of the words to your eyes.
In science, photons are used for more than just illumination.
“They’re ubiquitous,” says Richard Ruiz, a research associate at the Institute of Nuclear Physics in Krakow, Poland, and a theorist looking for new physics at the Large Hadron Collider. “Photons are everywhere in particle physics, so you almost forget about them.”
The photon has fueled centuries of discovery, and it remains an important tool today.
From wave, to particle, to boson
People have investigated the nature of light since ancient times, with early insights coming from philosophers and scholars in Egypt, Mesopotamia, India and Greece. Between the late 17th and early 20th centuries, scientists went back and forth on the answer to one question in particular: Does light behave as a particle or as a wave?
In 1690, Christiaan Huygens published Traité de la Lumière, his treatise on light. In it, he described light as being made up of waves that moved through the ether, which was thought to permeate space.
Isaac Newton declared in his 1704 book Opticks that he disagreed. When light reflects off of a surface, it acts like a bouncing ball; the angle at which it approaches the surface is equal to the angle at which it bounces off. Newton argued that this phenomenon, among other things, could be explained if light were made up of particles, which he called “corpuscules.”
A glass prism refracts a beam of white light into a rainbow of colors. Newton noticed that when the light was then refracted again, through a second prism, it did not divide any further; the rainbow colors stayed the same.
Newton said this could be explained by assuming that white light was made up of many different corpuscules of different sizes. Red light was made up of the biggest corpuscules; violet was made up of the smallest. Newton said their different sizes caused the corpuscules to be pulled through the glass at different, accelerated speeds. This spread them out, producing the rainbow of colors that could not be broken down further by a second prism.
Newton’s corpuscular model had a significant drawback, though.
When light travels through a small hole, it spreads out just like ripples in water. Newton’s corpuscular model couldn’t explain this behavior, and Huygens’ wave model could.
Still, scientists were generally inclined to dismiss Huygens and listen to Newton—he did write Principia, one of the most important books in the history of science, after all.
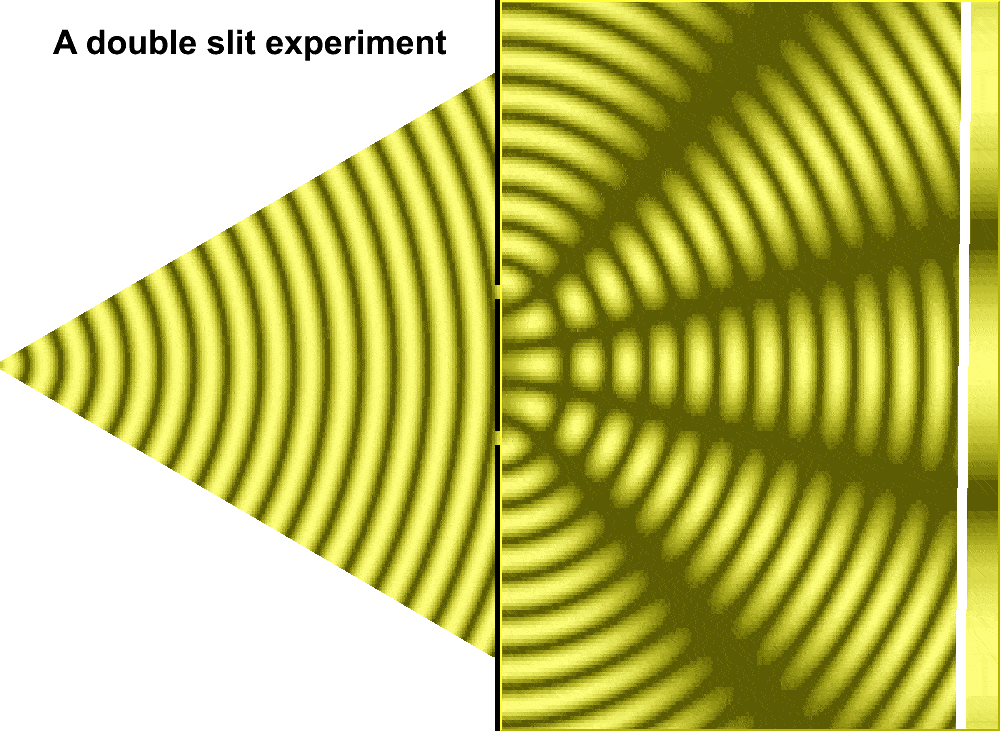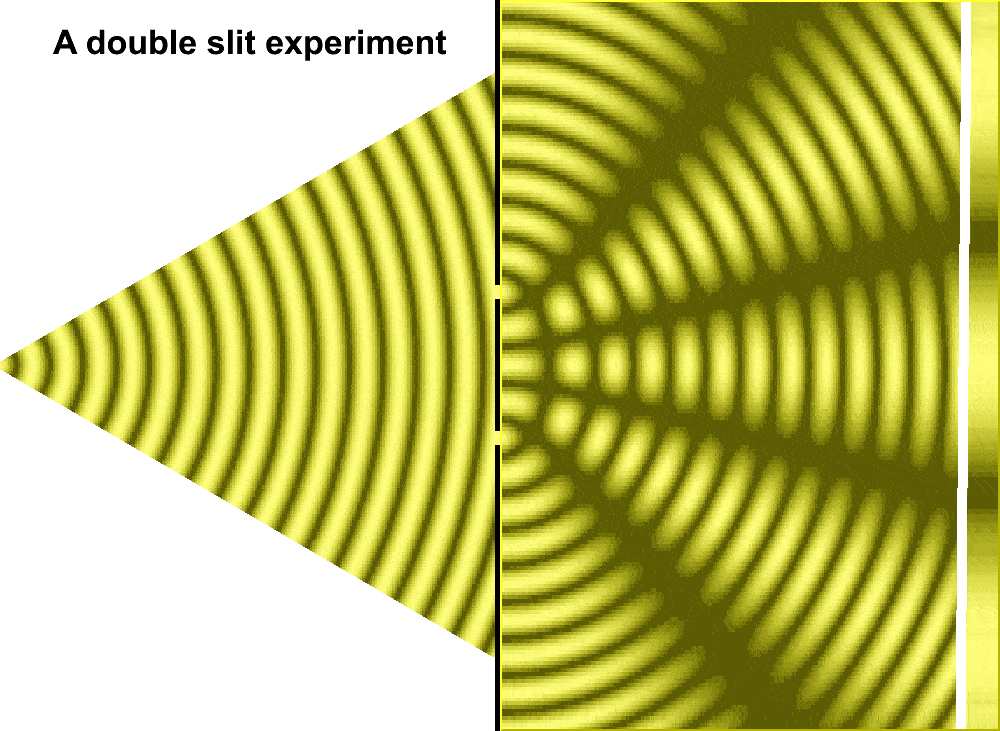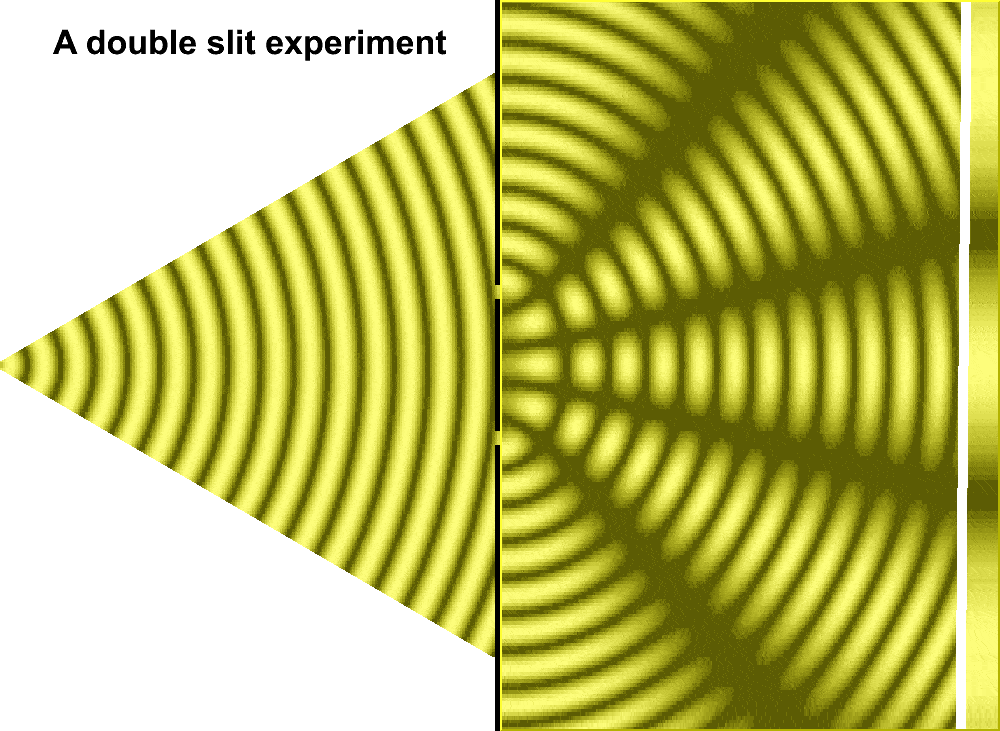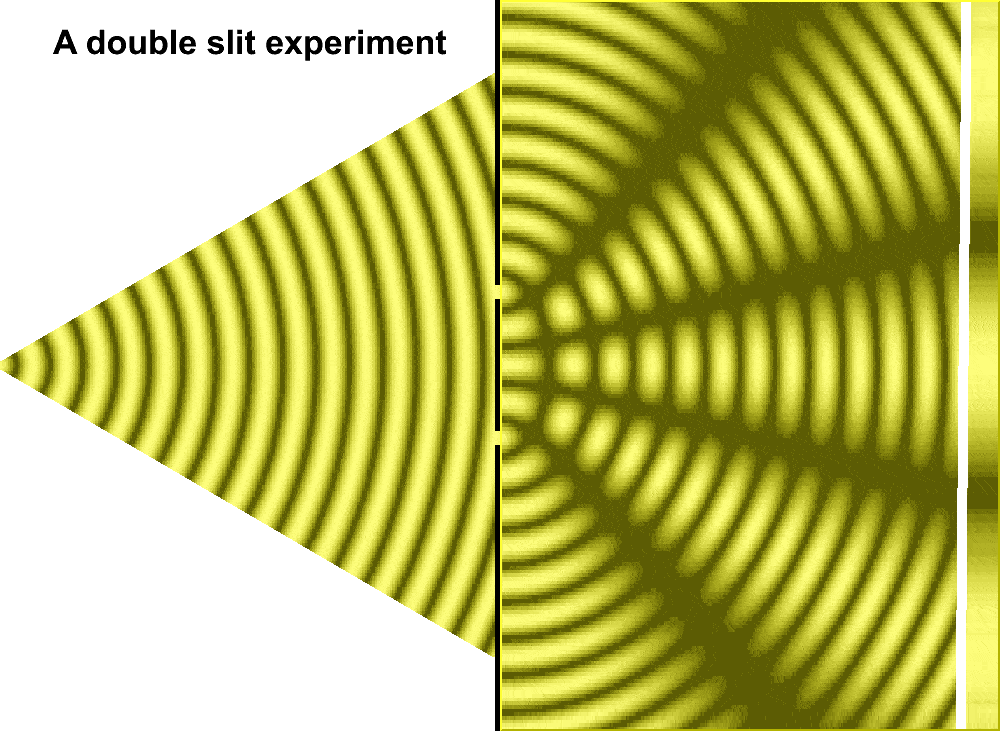
But Huygens’ model received some support in 1801, when Thomas Young conducted the double slit experiment. In the experiment, Young sent a beam of light through two small holes, side-by-side, and found that the light passing through them formed a particular pattern. At regular intervals the intersecting ripples emanating from the two holes interfered either constructively—combining to make brighter light—or destructively—canceling one another out. Just like waves.
About five decades later, another experiment put Huygens’ model definitively in the lead.
In 1850, Léon Foucalt compared the speed of light through air with the speed of light through water and found that, contrary to Newton’s assertions, light did not move faster in the denser medium. Instead, just like a wave would, it slowed down.
Eleven years later, James Clerk Maxwell published On Physical Lines of Force, in which he predicted the existence of electromagnetic waves. Maxwell noted their similarity to lightwaves, leading him to conclude that the two were one and the same.
It seemed that Huygens’ wave model had won the day. But in 1900, Max Planck came up with an idea that would spark a brand new concept of light.
Planck explained some puzzling behaviors of radiation by describing the energy of electromagnetic waves as divided into individual packets. In 1905, Albert Einstein built on Planck’s concept of energy packets and finally settled the corpuscule-versus-wave debate—by declaring it a tie.
As Einstein explained, light behaves as both a particle and a wave, with the energy of each particle of light corresponding to the frequency of the wave.
His evidence came from studies of the photoelectric effect—the way in which light knocked electrons loose from metal. If light traveled only in a continuous wave, then shining a light on metal for long enough would always dislodge an electron, because the energy the light transferred to the electron would accumulate over time.
But the photoelectric effect didn’t work that way. In 1902 Philipp Lenard had observed that only light above a certain energy—or lightwaves above a certain frequency—could pry an electron loose from the metal. And it seemed to do so on contact, immediately.
In this case, the light was acting more like a particle, an individual packet of energy.
Still convinced of the wave model of light, Robert Millikan set out to disprove Einstein’s hypothesis. Millikan took careful measurements of the relationship between the light and electrons involved in the photoelectric effect. To his surprise, he confirmed each of Einstein’s predictions.
Einstein’s study of the photoelectric effect earned him his sole Nobel Prize in 1921.
In 1923, Arthur Compton provided additional support for Einstein’s model of light. Compton aimed high-energy light at materials, and he successfully predicted the angles at which electrons released by the collisions would scatter. He did it by presuming the light would act like tiny billiard balls.
Chemist Gilbert Lewis came up with a name for these billiard balls. In a 1926 letter to the journal Nature, he called them “photons.”
The way that scientists think about photons has continued to evolve in more recent years. For one, the photon is now known as a “gauge boson.”
Gauge bosons are force-carrying particles that enable matter particles to interact via the fundamental forces. Atoms, for example, stick together because the positively charged protons in their nuclei exchange photons with the negatively charged electrons that orbit them—an interaction via the electromagnetic force.
Secondly, the photon is now thought of as a particle, a wave, and an excitation—kind of like a wave—in a quantum field.
A quantum field, such as the electromagnetic field, is a kind of energy and potential spread throughout space. Physicists think of every particle as an excitation of a quantum field.
“I like to think of a quantum field as a calm pond surface where you don’t see anything,” Ruiz says. “Then you put a pebble on the surface, and the water pops up a bit. That’s a particle.”
What Is a Photon in Physics?
Photons Are a «Bundle of Energy»
A photon is a particle of light defined as a discrete bundle (or quantum) of electromagnetic (or light) energy. Photons are always in motion and, in a vacuum (a completely empty space), have a constant speed of light to all observers. Photons travel at the vacuum speed of light (more commonly just called the speed of light) of c = 2.998 x 10 8 m/s.
Basic Properties of Photons
According to the photon theory of light, photons:
History of Photons
The term photon was coined by Gilbert Lewis in 1926, though the concept of light in the form of discrete particles had been around for centuries and had been formalized in Newton’s construction of the science of optics.
In the 1800s, however, the wave properties of light (by which is meant electromagnetic radiation in general) became glaringly obvious and scientists had essentially thrown the particle theory of light out the window. It wasn’t until Albert Einstein explained the photoelectric effect and realized that light energy had to be quantized that the particle theory returned.
Wave-Particle Duality in Brief
As mentioned above, light has properties of both a wave and a particle. This was an astounding discovery and is certainly outside the realm of how we normally perceive things. Billiard balls act as particles, while oceans act as waves. Photons act as both a wave and a particle all the time (even though it’s common but basically incorrect, to say that it’s «sometimes a wave and sometimes a particle» depending upon which features are more obvious at a given time).
Just one of the effects of this wave-particle duality (or particle-wave duality) is that photons, though treated as particles, can be calculated to have frequency, wavelength, amplitude, and other properties inherent in wave mechanics.
Fun Photon Facts
The photon is an elementary particle, despite the fact that it has no mass. It cannot decay on its own, although the energy of the photon can transfer (or be created) upon interaction with other particles. Photons are electrically neutral and are one of the rare particles that are identical to their antiparticle, the antiphoton.
Photons are spin-1 particles (making them bosons), with a spin axis that is parallel to the direction of travel (either forward or backward, depending on whether it’s a «left-hand» or «right-hand» photon). This feature is what allows for polarization of light.
Why do we say that photons are particles? [closed]
Want to improve this question? Add details and clarify the problem by editing this post.
This question may appear stupid but I really do have to understand. Maybe it’s just semantic and nothing else.
Why do we say that photons are (elementary) particles?
They are pure radiation, since they are massless, aren’t they? So they cannot be treated as point like particles, and I think that it’s a nonsense to think about them as particle according to the definition of a particle.
4 Answers 4
«Particles» with quotation marks because they are not classical billiard balls, they are quantum mechanical entities manifested in experiments microscopically with probability distributions.
The reason one calls photons and gluons and gravitons and Z and W particles is because of the validation of the standard model.
The above is the current state of particle physics, experiment and the theory that describes them and can predict new behaviors.
The photon emerged as a particle, at the time not separated from a classical particle because of the photoelectric effect. It was a proof that light was composed by quanta and these were named photons, to finally be called quantum mechanical entities, «particles». With quantum field theory the emergence of the classical electromagnetic radiation from the photon field is shown in this blog post of @Motl.
There is something called the Compton effect, where an electron and a photon interact with each other, and the scattering happens with a large, billiard-ball style change of momentum, rather than the «soft» sort of interaction you would expect from a fluid or continuous field.
All the elementary particles are described as excitations of quantum fields. What you think of as a particle is actually a much stranger object. It is an excitation in an operator field that spans all of spacetime.
The point of all this is that photons are described by quantum field theory in exactly the same way as all the other particles, so there is no reason to regard them as any different to the other particles. Photons are massless gauge vector bosons, and their behaviour is somewhat different to massive fermions like electrons, but these differences are all well described by quantum field theory.
What exactly is a photon? Definition, properties, facts
Let’s shine some light on the matter.
Imagine a shaft of yellow sunlight beaming through a window. According to quantum physics that beam is made of zillions of tiny packets of light, called photons, streaming through the air. But what exactly is a photon?
Photons are the stuff light is made of. Credit:JFC.
Definition
A photon is the smallest discrete amount or quantum of electromagnetic radiation. It is the basic unit of all light.
Photons are always in motion and, in a vacuum, travel at a constant speed to all observers of 2.998 x 10 8 m/s. This is commonly referred to as the speed of light, denoted by the letter c.
As per Einstein’s light quantum theory, photons have energy equal to their oscillation frequency times Planck’s constant. Einstein proved that light is a flow of photons, the energy of these photons is the height of their oscillation frequency, and the intensity of the light corresponds to the number of photons. Essentially, he explained how a stream of photons can act both as a wave and particle.
Photon properties
The basic properties of photons are:
History
The nature of light — whether you regard it as a particle or a wave — was one of the greatest scientific debates. For centuries philosophers and scientists have argued about the matter that was barely resolved a century ago.
The disciples of a sixth century BC branch of Hindu philosophy called Vaisheshika had a surprising physical intuition about light. Like the ancient Greeks, they used to believe the world was based on ‘atoms’ of earth, air, fire, and water. Light itself was thought to be made of such very fast-moving atoms called tejas. That’s remarkably similar to our modern theory of light and its composing photons, a term coined thousands of years later in 1926 by a chemist named Gilbert Lewis and an optical physicist called Frithiof Wolfers.
Later, around 300 BC, the ancient Greek physicist Euclid made a huge breakthrough when he posited light traveled in straight lines. Euclid also described the laws of reflection and, a century later, Ptolemy complemented with writings about refraction. IT wasn’t until 1021, however, that the laws of refraction were formally established in the seminal work Kitab al-Manazir, or Book of Optics, by Ibn al-Haytham.
The Renaissance would usher in a new age of scientific inquiry into the nature of light. Of note are René Descartes’ incursions in a 1637 essay called La dioptrique, where he argued that light is made of pulses that propagate instantaneously when contacting ‘balls’ in a medium. Later writing in Traité de la lumière published in 1690, Christiaan Huygens treated light as compressible waves in an elastic medium, just like sound pressure waves. Huygens showed how to make reflected, refracted, and screened waves of light and also explained double refraction.
By this time, scientists had split into two entrenched camps. One side believed that light was a wave while the other view was of light as particles or corpuscles. The great champion of the so-called ‘corpuscularists’ was none other than Isaac Newton, widely believed as the greatest scientist ever. Newton wasn’t fond at all of the wave theory since that would mean light would be able to stray too far into the shadow.
For much of the 18th century, corpuscular theory dominated the debate around the nature of light. But then, in May 1801, Thomas Young introduced the world to his now famous two-slit experiment where he demonstrated the interference of light waves.
Young’s slit experiment shows how each slit acts as a source of spherical waves, which “interfere” as they move from left to right as shown above. Credit: University of Louisville Department of Physics.
In the first version of the experiment, Young actually didn’t use two slits, but rather a single thin card. The physicist simply covered a window with a piece of paper with a tiny hole in it which served to funnel a thin beam of light. With the card in his hand, Young witnessed how the beam split in two. Light passing on one side of the card interfered with light from the other side of the card to create fringes, which could be observed on the opposite wall. Later, Young used this data to calculate the wavelengths of various colors of light and came remarkably close to modern values. The demonstration would provide solid evidence that light was a wave, not a particle.
Meanwhile, this time in France, the corpuscularist movement was gaining steam after recent developments attributed the polarization of light to some kind of asymmetry among the light corpuscles. They suffered a great defeat at the hand of Augustin Fresnel who in 1821 showed that polarization could be explained if light were a transverse wave with no longitudinal vibration. Previously, Fresnel also came up with a precise wave theory of diffraction.
By this point, there was little stable ground for Newton’s followers to continue the debate. It seemed light is a wave and that’s that. The problem was that the fabled aether — the mysterious medium required to support electromagnetic fields and to yield Fresnel’s laws of propagation — was missing despite everyone’s best efforts to find it. No one ever did, actually.
A huge breakthrough came in 1861 when James Clerk Maxwell condensed experimental and theoretical knowledge about electricity and magnetism in 20 equations. Maxwell predicted an ‘electromagnetic wave’, which can self-sustain, even in a vacuum, in the absence of conventional currents. This means no aether is required for light to propagate! Moreover, he predicted the speed of this wave to be 310,740,000 m s −1 — that’s just a few percent of the exact value of the speed of light.
“The agreement of the results seems to show that light and magnetism are affections of the same substance, and light is an electromagnetic disturbance propagated through the field according to electromagnetic laws”, wrote Maxwell in 1865.
From that day forward, the concept of light was united with those of electricity and magnetism for the first time.
On 14 December 1900, Max Planck demonstrated that heat radiation was emitted and absorbed in discrete packets of energy — quanta. Later, Albert Einstein showed in 1905 that this also applied to light. Einstein used the term Lichtquant, or quantum of light. Now, at the dawn of the 20th-century, a new revolution in physics would once again hinge on the nature of light. This time, it’s not about whether light is a crepuscule or wave. It’s whether it’s both or not.
Modern theory of light and photons
Einstein believed light is a particle (photon) and the flow of photons is a wave. The German physicist was convinced light had a particle nature following his discovery of the photoelectric effect, in which electrons fly out of a metal surface exposed to light. If light was a wave, that couldn’t have happened. Another puzzling matter is how photoelectrons multiply when strong light is applied. Einstein explained the photoelectric effect by saying that “light itself is a particle,” for which he would later receive the Nobel Prize in Physics.
The main point of Einstein’s light quantum theory is that light’s energy is related to its oscillation frequency. He maintained that photons have energy equal to “Planck’s constant times oscillation frequency,” and this photon energy is the height of the oscillation frequency while the intensity of light corresponds to the number of photons. The various properties of light, which is a type of electromagnetic wave, are due to the behavior of extremely small particles called photons that are invisible to the naked eye.
Einstein speculated that when electrons within matter collide with photons, the former takes the latter’s energy and flies out and that the higher the oscillation frequency of the photons that strike, the greater the electron energy that will come flying out. Some of you have a working proof of this idea in your very own home — it’s the solar panels! In short, he was saying that light is a flow of photons, the energy of these photons is the height of their oscillation frequency, and the intensity of the light is related to the number of photons.
More than a hundred years since Einstein showed the double nature of light, Swiss physicists at the École Polytechnique Fédérale de Lausanne captured the first-ever snapshot of this dual behavior. The team led by Fabrizio Carbone performed a clever experiment in 2015 in which a laser was used to fire onto a nanowire, causing electrons to vibrate. Light travels along this tiny wire in two possible directions, like cars on a highway. When waves traveling in opposite directions meet each other they form a new wave that looks like it is standing in place. Here, this standing wave becomes the source of light for the experiment, radiating around the nanowire. The fired a new beam of electrons to image the standing wave of light, which acts as a fingerprint of the wave-nature of light. The result can be seen below.
The first ever photograph of light as both a particle and wave. Credit: EPFL.
What a photon looks like
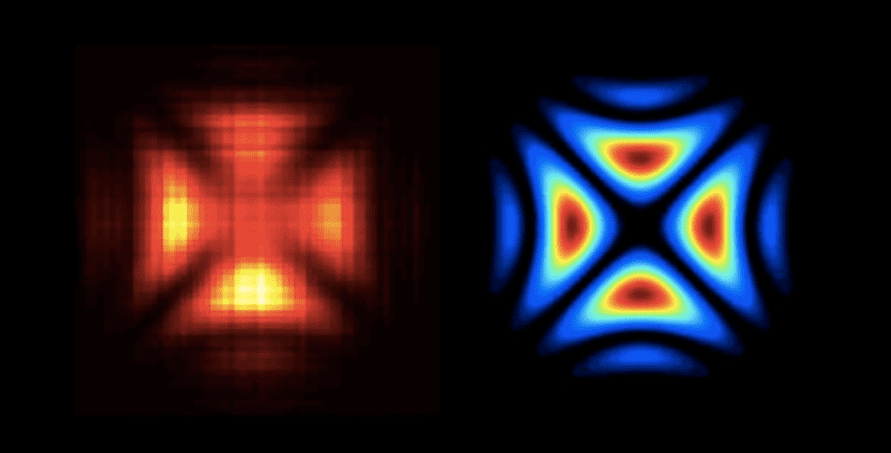
Have you ever wondered what shape does a photon have? Scientists have been pondering this question for decades and, finally, in 2016, Polish physicists created the first ever hologram of a single light particle. The team at the University of Warsaw made the hologram by firing two light beams at a beamsplitter, made of calcite crystal, at the same time. The beamsplitter is akin to a traffic light intersection so each photon can either pass straight through or make a turn. When a photon is on its own, each path is equally probable but when more photons are involved they interact and the odds change. If you know the wave function of one of the photons, it’s possible to figure out the shape of the second from the positions of flashes appearing on a detector. The resulting image looks a bit like a Maltese cross, just like the wave function predicted from Schrödinger’s equation.
Hologram of a single photon reconstructed from raw measurements seen in the left-hand side versus the theoretically predicted photon shape on the right-hand side. Credit: FUW






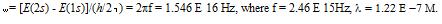
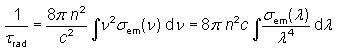
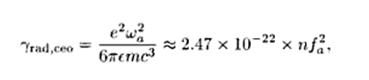
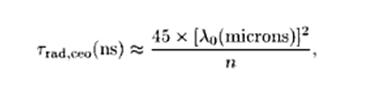

:max_bytes(150000):strip_icc()/GettyImages-180294159-57a0b3915f9b589aa9b765e2.jpg)
:max_bytes(150000):strip_icc()/AZJFaceShot-56a72b155f9b58b7d0e783fa.jpg)