What is acid rain
What is acid rain
What is acid rain and how is it formed?
How is acid rain formed?
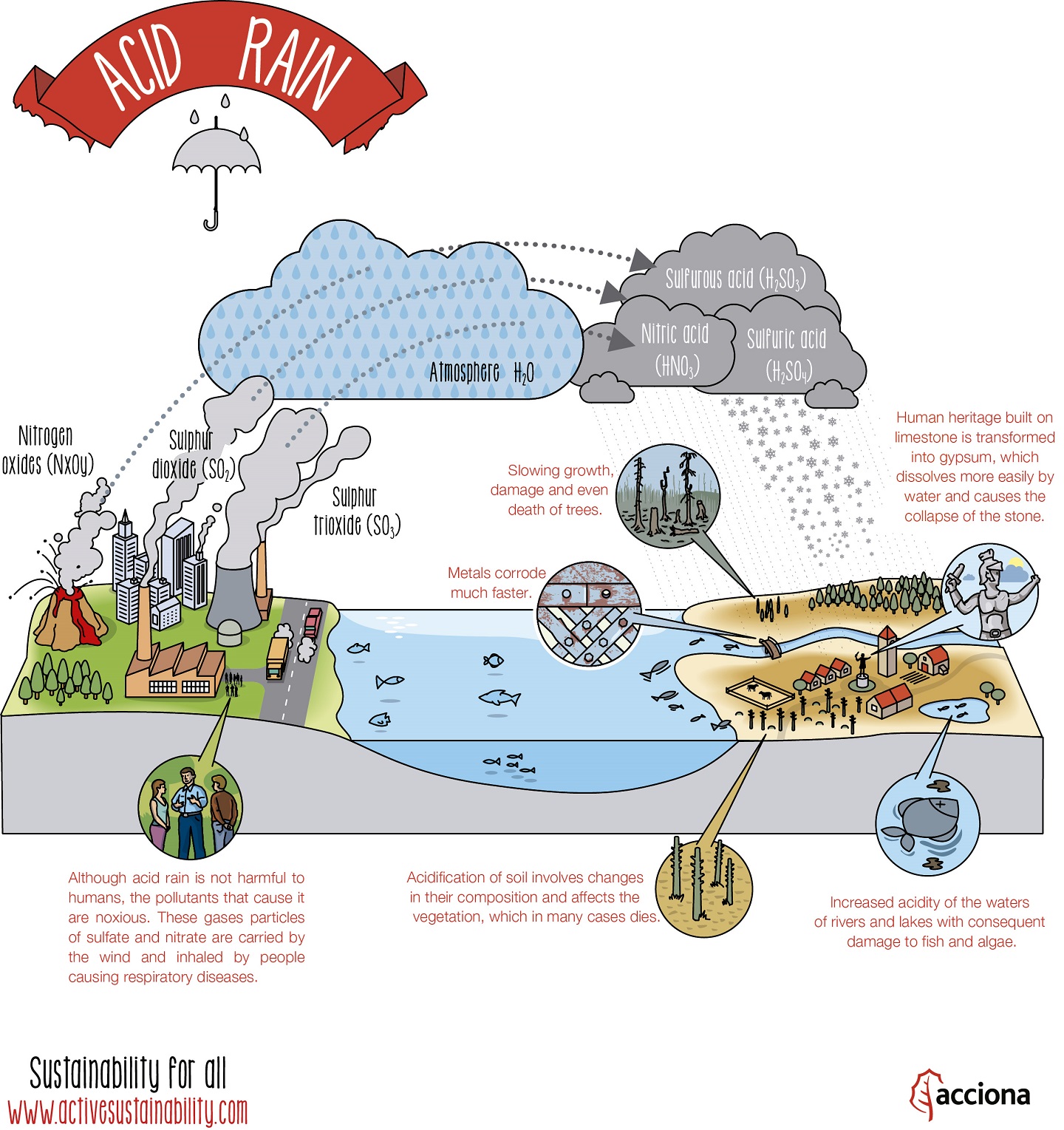
Acid rain is one of the consequences of air pollution. It occurs when emissions from factories, cars or heating boilers contact with the water in the atmosphere. These emissions contain nitrogen oxides, sulfur dioxide and sulfur trioxide, which when mixed with water become sulfurous acid, nitric acid and sulfuric acid. This process also occurs naturally through volcanic eruptions.
Acid rain effects
Is acid rain harmful to humans?
Acid rain itself is not harmful to humans, ie, the skin contact with contaminated water or snow does not pose a health risk. However, the gases that cause this rain (nitrogen oxides, sulfur dioxide and sulfur trioxide), are harmful. These gases contain particles of sulfate and nitrate and are carried by the wind and inhaled by people causing respiratory diseases.
How to stop acid rain?
The only way to stop acid rain is to reduce emissions that cause it. This involves betting on renewable energy sources and reducing the use of fossil fuels in the industrial and automotive sector and in the daily life of every citizen.
The following infographic might help better understand what acid rain is and what are its effects:
Share on social media
How is our planet?
Impacts of climate change
«How to avoid climate change?»
The three pillars of sustainable cities and communities
A climate ticket to promote sustainable mobility
Ten questions and answers about green hydrogen
SUBSCRIBE TO OUR NEWSLETTER
. and stay informed with the latest news on innovation.
Información sobre protección de datos
En cumplimiento del Reglamento UE 2016/679 de Protección de Datos y demás normativa vigente en materia de Protección de Datos, se le informa de que sus datos de carácter personal serán tratados por Acciona, S.A. (en adelante, ACCIONA), con los siguientes datos identificativos NIF: A08001851, Dirección: Avenida de Europa, 18, Parque empresarial de la Moraleja, 28108 de Alcobendas (Madrid), Tel: +34 91 663 28 50, email: protecciondedatos@acciona.com, con la finalidad de atender sus comentarios y gestionar sus consultas, solicitudes, reclamaciones o sugerencias, así como el envío, por medios electrónicos, de información sobre nuestros servicios y productos, a través del correo electrónico de contacto.
La base jurídica para el tratamiento de los datos es el consentimiento del usuario al comunicarse con nosotros.
Los datos se conservarán mientras se mantenga la relación y no se solicite su supresión y, en cualquier caso, nunca durante un plazo superior a doce meses.
En caso de que su petición no sea dirigida a ACCIONA, sino a una entidad que forma parte del Grupo Acciona, ésta comunicará los datos a la sociedad del Grupo que pueda atender su solicitud de servicio o información de forma más eficiente. En este sentido, la comunicación de estos datos puede constituir una transferencia internacional, por estar estas empresas ubicadas en países fuera del territorio de la Unión Europea, para poder atender las necesidades de comunicación entre las personas que forman parte del Grupo a nivel mundial. (Puede consultar un listado de empresas del Grupo en www.acciona.com/es/accionistas-inversores/informacion-financiera/cuentas-anuales). La aceptación de los términos de la privacidad supone el consentimiento para la transferencia internacional de sus datos necesaria para la correcta tramitación de su petición. No están previstas otras cesiones de datos, salvo obligación legal.
El interesado puede ejercitar sus derechos de acceso, rectificación, supresión, portabilidad y la limitación u oposición, ante Acciona, S.A. dirigiéndose por escrito al Departamento de Protección de datos sita en Avenida de Europa, 18, 28108 de Alcobendas (Madrid) o mediante el envío de un correo electrónico en la siguiente dirección: protecciondedatos@acciona.com, adjuntando en ambos casos copia del DNI u otro documento identificativo. Asimismo, podrá en cualquier momento, retirar el consentimiento prestado dirigiéndose a la dirección arriba indicada, así como reclamar ante la Autoridad de Control (Agencia Española de Protección de Datos www.aepd.es).
Para más información ponemos a su disposición la Política de Privacidad de la Página.
Sustainability is understood as the development that meets the present needs without compromising the capacities of future generations, ensuring the balance between economic growth, environmental care and social welfare. In Sustainability for all we promote the awareness and difussion of good practices that allow to combine economic and social development with the preservation of natural resources.
What is Acid Rain?
Acid rain, or acid deposition, is a broad term that includes any form of precipitation with acidic components, such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. This can include rain, snow, fog, hail or even dust that is acidic.
What Causes Acid Rain?
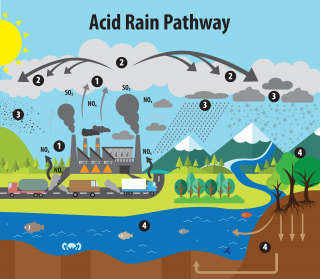
Acid rain results when sulfur dioxide (SO2) and nitrogen oxides (NOX) are emitted into the atmosphere and transported by wind and air currents. The SO2 and NOX react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
While a small portion of the SO2 and NOX that cause acid rain is from natural sources such as volcanoes, most of it comes from the burning of fossil fuels. The major sources of SO2 and NOX in the atmosphere are:
Winds can blow SO2 and NOX over long distances and across borders making acid rain a problem for everyone and not just those who live close to these sources.
Forms of Acid Deposition
Wet Deposition
Wet deposition is what we most commonly think of as acid rain. The sulfuric and nitric acids formed in the atmosphere fall to the ground mixed with rain, snow, fog, or hail.
Dry Deposition
Acidic particles and gases can also deposit from the atmosphere in the absence of moisture as dry deposition. The acidic particles and gases may deposit to surfaces (water bodies, vegetation, buildings) quickly or may react during atmospheric transport to form larger particles that can be harmful to human health. When the accumulated acids are washed off a surface by the next rain, this acidic water flows over and through the ground, and can harm plants and wildlife, such as insects and fish.
The amount of acidity in the atmosphere that deposits to earth through dry deposition depends on the amount of rainfall an area receives. For example, in desert areas the ratio of dry to wet deposition is higher than an area that receives several inches of rain each year.
Measuring Acid Rain
Policymakers, research scientists, ecologists, and modelers rely on the National Atmospheric Deposition Program’s (NADP) National Trends Network (NTN) for measurements of wet deposition. The NADP/NTN collects acid rain at more than 250 monitoring sites throughout the US, Canada, Alaska, Hawaii and the US Virgin Islands. Unlike wet deposition, dry deposition is difficult and expensive to measure. Dry deposition estimates for nitrogen and sulfur pollutants are provided by the Clean Air Status and Trends Network (CASTNET). Air concentrations are measured by CASTNET at more than 90 locations.
When acid deposition is washed into lakes and streams, it can cause some to turn acidic. The Long-Term Monitoring (LTM) Network measures and monitors surface water chemistry at over 280 sites to provide valuable information on aquatic ecosystem health and how water bodies respond to changes in acid-causing emissions and acid deposition.
Acid rain, explained
The fossil fuels that humans burn for energy can come back to haunt us as acid rain.
What is Acid Rain?
Acid rain describes any form of precipitation that contains high levels of nitric and sulfuric acids. It can also occur in the form of snow, fog, and tiny bits of dry material that settle to Earth. Normal rain is slightly acidic, with a pH of 5.6, while acid rain generally has a pH between 4.2 and 4.4.
Causes of acid rain
Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a product of human activities. The biggest sources are coal-burning power plants, factories, and automobiles.
When humans burn fossil fuels, sulfur dioxide (SO2) and nitrogen oxides (NOx) are released into the atmosphere. Those air pollutants react with water, oxygen, and other substances to form airborne sulfuric and nitric acid. Winds may spread these acidic compounds through the atmosphere and over hundreds of miles. When acid rain reaches Earth, it flows across the surface in runoff water, enters water systems, and sinks into the soil.
A virtual tree graveyard of Norway spruce in Poland bears the scars of acid rain. Caused when rain droplets absorb air pollution like sulfur and nitrogen oxides, acid rain weakens trees by dissolving nutrients in the soil before plants can use them.
Effects of acid rain
Sulfur dioxide and nitrogen oxides are not primary greenhouse gases that contribute to global warming, one of the main effects of climate change; in fact, sulfur dioxide has a cooling effect on the atmosphere. But nitrogen oxides contribute to the formation of ground-level ozone, a major pollutant that can be harmful to people. Both gases cause environmental and health concerns because they can spread easily via air pollution and acid rain.
Acid rain has many ecological effects, especially on lakes, streams, wetlands, and other aquatic environments. Acid rain makes such waters more acidic, which results in more aluminum absorption from soil, which is carried into lakes and streams. That combination makes waters toxic to crayfish, clams, fish, and other aquatic animals. (Learn more about the effects of water pollution.)
Some species can tolerate acidic waters better than others. However, in an interconnected ecosystem, what affects some species eventually affects many more throughout the food chain, including non-aquatic species such as birds.
Acid rain and fog also damage forests, especially those at higher elevations. The acid deposits rob the soil of essential nutrients such as calcium and cause aluminum to be released in the soil, which makes it hard for trees to take up water. Trees’ leaves and needles are also harmed by acids.
What is acid rain
What is Acid Rain?
How acidic is acid rain?
Acidity is measured using a scale called the pH scale. This scale goes from 0 to 14. 0 is the most acidic and 14 is the most alkaline (opposite of acidic). Something with a pH value of 7, we call neutral, this means that it is neither acidic nor alkaline.
Very strong acids will burn if they touch your skin and can even destroy metals. Acid rain is much, much weaker than this, never acidic enough to burn your skin.
Rain is always slightly acidic because it mixes with naturally occurring oxides in the air. Unpolluted rain would have a pH value of between 5 and 6. When the air becomes more polluted with nitrogen oxides and sulphur dioxide the acidity can increase to a pH value of 4. Some rain has even been recorded as being pH2.
The Effects of Acid Rain
Acid rain can be carried great distances in the atmosphere, not just between countries but also from continent to continent. The acid can also take the form of snow, mists and dry dusts. The rain sometimes falls many miles from the source of pollution but wherever it falls it can have a serious effect on soil, trees, buildings and water.
Forests all over the world are dying, fish are dying. In Scandinavia there are dead lakes, which are crystal clear and contain no living creatures or plant life. Many of Britain’s freshwater fish are threatened, there have been reports of deformed fish being hatched. This leads to fish-eating birds and animals being affected also. Is acid rain responsible for all this? Scientists have been doing a lot of research into how acid rain affects the environment.
It is thought that acid rain can cause trees to grow more slowly or even to die but scientists have found that it is not the only cause. The same amount of acid rain seems to have more effect in some areas than it does in others.
Other soils are already slightly acidic and these are particularly susceptible to the effects of acid rain.
Acid rain can effect trees in several different ways, it may:
• dissolve and wash away the nutrients and minerals in the soil
which help the trees to grow.
• cause the release of harmful substances such as aluminium into the soil.
• wear away the waxy protective coating of leaves, damaging them
and pre venting them from being able to photosynthesise properly.
A combination of these effects weakens the trees which means that they can be more easily attacked by diseases and insects or injured by bad weather. It is not just trees that are affected by acid rain, other plants may also suffer.

Lakes and Rivers
As the acidity of a lake increases, the water becomes clearer and the numbers of fish and other water animals decline. Some species of plant and animal are better able to survive in acidic water than others. Freshwater shrimps, snails, mussels are the most quickly affected by acidification followed by fish such as minnows, salmon and roach. The roe and fry (eggs and young) of the fish are the worst affected, the acidity of the water can cause deformity in young fish and can prevent eggs from hatching properly.
The acidity of the water does not just affect species directly, it also causes toxic substances like aluminium to be released into the water from the soil, harming fish and other aquatic animals.
Lakes, rivers and marshes each have their own fragile ecosystem with many different species of plants and animals all depending on one another to survive. If a species of fish disappears, the animals which feed on it will gradually disappear too. If the extinct fish used to feed on a particular species of large insect, that insect population will start to grow, this in turn will affect the smaller insects or plankton on which the larger insect feeds.


Every type of material will become eroded sooner or later by the effects of the climate. Water, wind, ice and snow all help in the erosion process but unfortunately, acid rain can help to make this natural process even quicker. Statues, buildings, vehicles, pipes and cables can all suffer. The worst affected are things made from limestone or sandstone as these types of rock are particularly susceptible and can be affected by air pollution in gaseous form as well as by acid rain.
Where is it coming from?
Until relatively recently air pollution has been seen as a local issue. It was in southern Scandinavia in the late 1950’s that the problems of acid rain were first observed and it was then that people began to realise that the origins of this pollution were far away in Britain and Northern Europe. One early answer to industrial air pollution was to build very tall chimneys. Unfortunately all this does is push the polluting gases up into the clouds allowing emissions to float away on the wind. The wind carries the pollution many hundreds of miles away where it eventually falls as acid rain. In this way Britain has contributed at least 16% of the acid deposition in Norway. Over ninety percent of Norway’s acid pollution comes from other countries. The worst European polluters are Germany, UK, Poland and Spain, each of them producing over a million tons of sulphur emissions in 1994. Governments are now beginning to admit that acid rain is a serious environmental problem and many countries are now taking steps to reduce the amount of sulphur and nitrogen emissions.
What can be done?
• Burning fossil fuels is still one of the cheapest ways to produce electricity so people are now researching new ways to burn fuel which don’t produce so much pollution.
• Governments need to spend more money on pollution control even if it does mean an increase in the price of electricity.
• Sulphur can also be ‘washed’ out of smoke by spraying a mixture of water and powdered limestone into the smokestack.
• Cars are now fitted with catalytic converters which remove three dangerous chemicals from exhaust gases.
Find alternative sources of energy
• Governments need to invest in researching different ways to produce energy.
• Two other sources that are currently used are hydroelectric and nuclear power. These are ‘clean’ as far as acid rain goes but what other impact do they have on our environment?
• Other sources could be solar energy or windmills but how reliable would these be in places where it is not very windy or sunny?
• All energy sources have different benefits and costs and all theses have to be weighed up before any government decides which of them it is going to use.
• Greater subsidies of public transport by the government to encourage people to use public transport rather than always travelling by car.
• Walking, cycling and sharing cars all reduce the pollution from vehicles


Restoring the Damage done by Acid Rain
What is Acid Rain?
Acid rain, or acid deposition, is defined or a term that includes any form of precipitation with acidic components, like sulphuric or nitric acid, that falls from the atmosphere in wet or dry forms, including rain, snow, fog, hail and dust.
What Causes Acid Rain?
Acid rain is a result of sulphur dioxide ( SO2) and nitrogen oxides (NOX) being emitted into the atmosphere and transported by wind and air, making it a problem for all, not just those who live near the sources of the pollution. Acid rain consists of sulphuric and nitric acids, formed when aforementioned gases mix and react with water, oxygen and other chemicals, which is then mix with water again and other materials before falling to the ground.
We believe in Compassionate Humanity
We believe in One Planet Thriving
We love our Home
As stated, acid deposition can occur in wet and dry forms. When wet, it is what we most commonly think of as acid rain. This falls as rain, snow, fog or hail. Dry deposition occurs when acidic particles and gases may deposit on surfaces (water bodies, vegetation, buildings) quickly or may react during atmospheric transport to form larger particles that can pose a risk to human health. When these accumulated acids are washed off the surface by the next rain, the acidic water flows over and through the ground and could be harmful to plants and wildlife. The amount of acidity in the atmosphere that occurs through dry deposition depends on the amount of rainfall an area receives.
What are the Effects of Acid Rain?
While sulphur dioxides actually have a cooling effect on the atmosphere, nitrogen oxides contribute to the formation of ozone, which can be harmful to people and plants. Both of these gases are causes for concern because they can spread easily via air pollution and acid rain.
While some species can handle acidic waters better than others, in connected ecosystems, there will be a domino effect throughout the food chain, whereby one species dying off because it cannot tolerate acidic rain will leave another without food, killing that species off as well.
Acid rain and fog also damage forests, especially those at higher elevations. The acid deposits rob the soil of nutrients such as calcium and cause aluminium to be released into the soil, which makes it difficult for trees to suck up water. The results are less healthy trees and planets that are more vulnerable to cold temperatures, insects and disease. Additionally, the trees’ ability to reproduce is compromised.
Physical structures such as limestone buildings and cars can also be affected by acid rain. When it takes the form of fog, it can be inhaled and cause health problems, like eye irritation and asthma.
How Do We Measure It?
Acid rain is measured using a pH scale for which 7.0 is neutral. The lower a substance’s pH (less than 7), the more acidic it is; the higher it is (greater than 7), the more alkaline it is.
Normally, rain has a pH of about 5.6, making it slightly acidic because CO2 dissolves into it, forming a weak carbonic acid. However, normal precipitation reacts with alkaline chemicals that can be found in air, soils, bedrock, lakes and streams, the reactions of which usually neutralise natural acids. Acid rain has a pH of between 4.2 and 4.4.
When acid deposition is washed into lakes and streams, it can cause some to turn acidic.
How Do We End It?
Unfortunately, the only way to reverse the occurrence of acid rain is to curb the release of the pollutants that cause it, meaning that we need to burn fewer fossil fuels and set effective air quality standards.
In the US, the Clean Air Act of 1990 implemented pollution limits that helped cut SO2 emissions by 88% between 1990 and 2017. Additionally, air quality standards have also lowered nitrogen dioxide emissions by 50% in the same time period.
Landscapes that have been affected by acid rain can recover from its damage, however it takes time.